Surface Tension and Viscosity Are Used to Describe
In this paper we describe the simultaneous measurement of surface tension and viscosity using freely decaying shape oscillations of acoustically levitated droplets of different liquids silicone oils AK 5 and AK 10 squalane 1-propanol 1-butanol 1-pentanol 1-hexanol 1. The key liquid characteristics are varied in the following ranges.

Chapter 11 Surface Tension And Viscosity Ppt Video Online Download
Surface tension measurements are carried out when new coating formulations are developed or when the quality of a detergent is being evaluated.

. Surface tension is determined by the difference in interactions between liquid molecules with the molecules of the liquid in contact. Viscosity is a quantity of fundamental importance in fluid transport problems as well as in issues concerning reaction kinetics in melt processing. Surface tension is the energy required to increase the surface area of a liquid by a given amount.
The two are equivalent but when referring to energy per unit of area it is common to use the term surface energy which is a more general term in the sense that it applies also to solids. Surface tension Viscosity is dependent on molecular interactions. By waving a bar magnet in front of the paper clip I am applying a magnetic force on it which causes the paper clip to move.
The stronger the intermolecular interactions the greater the surface tension. Chemistry questions and answers. Surface tension pressure and viscosity are all phenomenological parameters used to describe matter at the macroscopic scale.
There is no significant drag on the paper clip except that from the bulk water phase below the air phase is assumed to have nearly zero viscosity. It seems like both involves the performance of molecules. Surface tension is an important factor in the phenomenon of capillarity.
Surface tension capillary action and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Dynamic viscosity 0001400063 Pas. Surface tension has the dimension of force per unit length or of energy per unit area.
Density and viscosity of pyridinium-based ionic liquids and their binary mixtures with water at several temperatures By fatemeh Sadeghian Density dynamic viscosity and derived properties of binary mixtures of methanol or ethanol with water ethyl acetate and methyl acetate at T29315 29815 and 30315K. Water glycerol Diesel fuel stabilizers and emulsifiers are. From secondary sources to assess the limitations of calorimetry experiments and design modifications to equipment used describe dissolutions which release heat as exothermic and give examples.
Whereas surface tension is determined by the difference of interactions between the molecules of the material fluid. Surface tension viscosity 2 The wide distribution and importance of water on. Up to 10 cash back Several modeling and experimental investigations 25262728 have been conducted on viscosity and surface tension of alcohol DMSO binary systems.
Results from the surface tension experiments with flour indicated that flour solutions with a higher viscosity than water had lower or about the same surface tension as water Figure 2B. Properties such as surface tension and dynamic viscosity. Surface tension of water was not statistically different from that of the agar solutions p-values of the students t-test are above 005 Table 1.
Additionally the more polar the molecule the more viscous. Explains three different methods to measure surface tension. Equations were used to calculate the surface tension and viscosity respectively.
Interfacial tension 000341004257 Nm. 1 Describe the properties of water that accounts for its surface tension viscosity liquid state at ambient temperature and solvent power2 What is the importance of the terms polar and nonpolar3 What are salt bridges4Describe what buffers do how they do it and the conditions under which a buffer. Surface tension 00275007269 Nm.
Biology questions and answers. It is responsible for giving drops their round appearance. The viscosity of molten systems often dictates the castability or ability to fill a mold cavity of many metals and their alloys1.
The surface tension increases as the molecular weight of the molecule increases. Viscosity and surface tension. Although they all depend on the intermolecular interactions there are some very fundamental differences.
Vaporization Which response has the following substances arranged in order of increasing boiling point. Surface tension capillary action viscosity vapor pressure c. Surface tension is influenced by the cohesive forces of the molecules and viscosity is related to the shear stress in the solution.
Surface and interfacial tension play a key role in several industrial processes including for example detergents coatings and oil recovery to name a few. Ar NaClO_3 H2O H_2Se NaCIO_3 H_2O H_2Se Ar NaClO_3 H_2SC H_2O Ar Ar. Substances with large amounts of H bonds have high surface tensions.
1 γ soln n solv n soln d soln d solv γ solv where γ soln is the surface tension of the solution γ solv is the surface tension of the solvent n solv is the number of drops of solvent n soln is the number of drops of solution d soln is the solution density and d solv is the solvent density and. Viscosity And Surface Tension However viscosity results due to collaboration among molecules of the identical molecules located in the same material. The term used to describe resistance to flow of a liquid is.
11-15-99 Sections 1010 - 1013 Real-life fluids. Complete the table by descibing the types of intermolecular forces and arrange these types according to increasing streanth by numbering 1-5 Types of molecular forces Description of the types of intermolecular forces strength of intermolecular forces Ion-ion interaction ion-dipole interaction dipole-dipole interaction hydrogen bonds london dispersion force. Viscosity occurs because of an alliance between identical molecules located in the same liquid material.
Real-life fluids like air water oil blood shampoo or anything like that often dont perfectly obey the fairly straight-forward Bernoullis principle and in some cases Bernoullis principle doesnt really come close to describing the behavior of real-life fluids when theyre flowing in real-life situations. Additionally the more polar the molecule the higher the surface tension. The rules governing surface tension are the same as for viscosity.
The paper clip doesnt fall because surface tension holds it in place. Viscosity is related to a liquids resistance to being deformed or moved. Surface tension viscosity 2 the wide distribution and.
Surface tension is the downward toward the middle force that a fluid exerts on its surface molecules. Only one of them used density-based models and equations of state so this study has been dedicated to reliable models of viscosity and surface tension based on the equation of. The viscosity increases as the molecular weight size of the molecules increases.
Surface tension is the property of a liquids surface that resists force and itis caused by unbalanced forces on surface molecules that pull toward the main part of the liquid.

Viscosity Surface Tension And Temperature Science Project Education Com

Is It Viscosity Or Is It Surface Tension Ctg Technical Blog

Viscosity Surface Tension Definition With Examples Of Viscosity Surface Tension

1 3 Intermolecular Forces In Action Surface Tension Viscosity And Capillary Action Chemistry Libretexts

Viscosity Surface Tension And Temperature Science Project Education Com
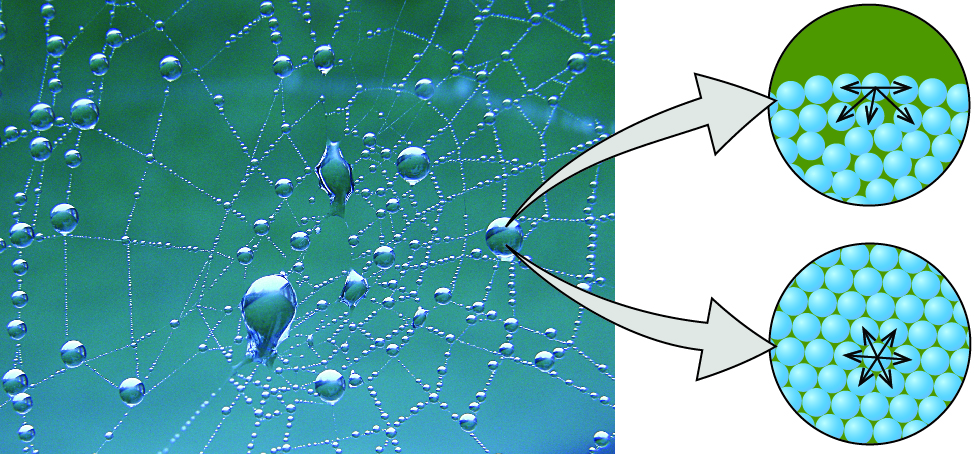
7 1 Surface Tension Viscosity And Capillary Action Chemistry Libretexts

Surface Tension Definition Examples Applications Of Surface Tension

Mechanical Properties Of Fluids Definition Streamline Flow Surface Tension Bernoulli S Principle

Viscosity And Surface Tension Of Acetone Water Solutions Versus Acetone Download Scientific Diagram

Viscosity Surface Tension And Temperature Science Project Education Com

Viscosity Surface Tension And Temperature Science Project Education Com

Surface Tension What Is Surface Tension Kibron

Viscosity Surface Tension Definition With Examples Of Viscosity Surface Tension
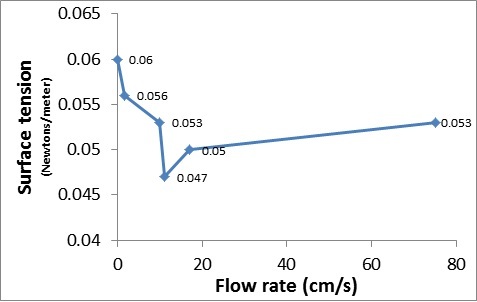
On The Relationship Between Viscosity And Surface Tension Journal Of Emerging Investigators
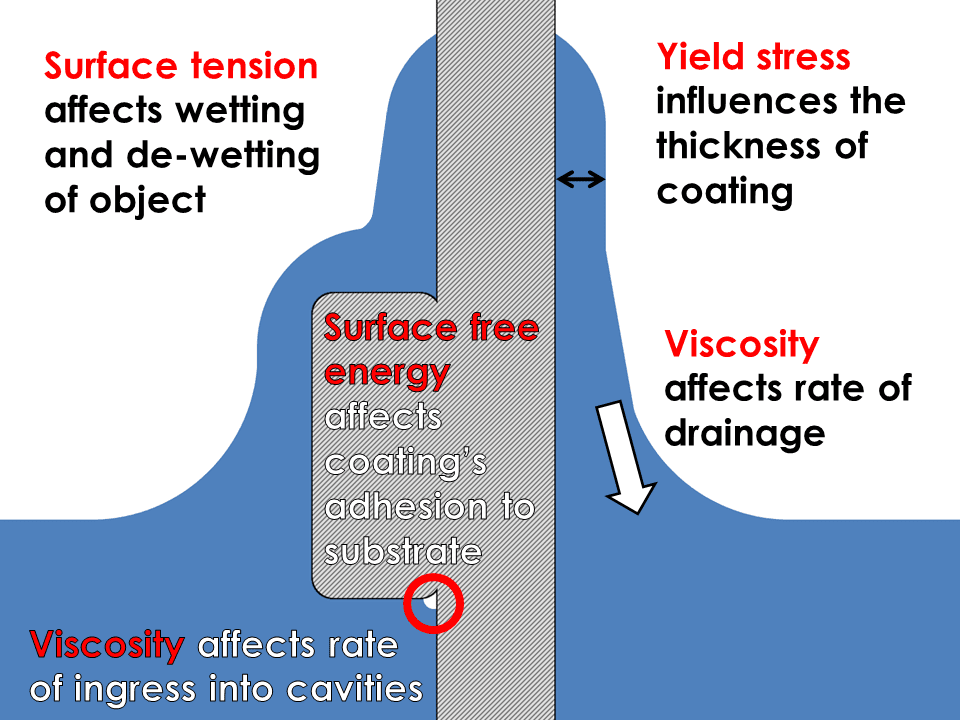
Dip Coating Viscosity Yield Stress And Surface Tension Rheology Lab
A Surface Tension Of The Ethanol Water Mixture As A Function Of Download Scientific Diagram

Solved 7 The Surface Tension And Viscosity Of Water At Chegg Com
How Are Viscosity And Intermolecular Forces Related Quora

Chapter 11 Surface Tension And Viscosity Ppt Video Online Download
Comments
Post a Comment